COVID-19, increased risk of blood clots and possible preventive and curative measures
Hyperactivation of platelets and the coagulation system, development of abnormally coagulation (hypercoagulability) due to COVID-19, and the risk of thrombosis.
The coronavirus epidemic was declared a pandemic by the World Health Organization (WHO) on March 11, 2020, slowly taking its toll for 2 years. The primary focus was on reducing the spread of the virus and treating respiratory symptoms, but over time, the neurological and hemostatic effects of COVID-19 on the hemostatic system become increasingly apparent. Uncontrolled thrombosis plays an important role in the pathogenesis of the coronavirus disease (COVID-19) caused by the SARS-CoV-2 virus.
Clinical data suggest that platelet hyperactivity plays a role in the pathology of COVID-19 from the outset and that platelets may play a critical role in the progression of COVID-19. Hyperactivation of platelets and the coagulation system appears to be an important driver of inflammation and may be associated with increased production of inflammatory proteins induced by severe cases of COVID-19, in which disseminated intravascular coagulation and platelet hyperactivity are associated with poor prognosis and an increased risk of death.1
- A cytokine storm is an overly inflammatory reaction of the immune system that can lead to organ failure and eventually complete collapse of the body. Cytokines are molecules that serve to transfer information between immune cells. Normally, they regulate the strength of the immune response against a pathogen. The sudden, large release of cytokines is called a cytokine storm. When someone develops a cytokine storm, their body produces too much protein, which causes inflammation.In such cases, it is no longer the pathogen that damages the organs, but the too strong immune response that completely consumes the body's own energies.Covid-19 can also have a detrimental effect on the vascular system, as this strong inflammatory response increases the risk of blood clots, leading to heart attack and thrombosis.The term became the subject of everyday speech during the coronavirus epidemic of 2020, when it became clear that the severe condition or death of those infected was often not caused by the virus itself or by pneumonia, but by a cytokine storm.(Health Guide)
- Disseminated intravascular coagulation refers to the abnormal activation of the coagulation system within the vascular system.Disseminated intravascular coagulation (DIC) occurs when activation is released from the beneficial framework of the inhibitory system and clot formation is initiated (disseminated) throughout the vascular system.Loss of blood clotting consumes normally available clotting proteins and thus causes severe bleeding.In practice, this means that abnormal coagulation and abnormal bleeding occur simultaneously.(Home Pharmacy)
While many people with COVID-19 develop mild to moderate symptoms, some develop profound, seemingly uncontrolled inflammatory reactions that lead to acute lung injury and hypoxemic (low oxygen in the blood) respiratory failure, the most common cause of death. The interaction between inflammation and blood clotting - thrombo-inflammation - has been well described and recently reviewed.2, 3
COVID-19 infection is associated with coagulation disorders characterized by elevated levels of procoagulant factors (including fibrinogen essential for coagulation) and increased levels of D-dimers, which are associated with higher mortality.4, 5
- Procoagulant: a substance that is needed for the blood to coagulate.
- Fibrinogen: causes the formation of fibrin fibers, which form a network of fibrins that, together with the adherent platelets, form a blood clot.
- D-dimer is a protein product that is formed during the clotting process.The D-dimer test is the most common and reliable laboratory method for confirming venous thrombosis and embolism.It shows that the clotting process has started in the blood circulating inside the vascular system.
Recently published and other reports mention an increased incidence of venous thromboembolic events (VTE) in critically ill patients admitted to the intensive care unit (ICU) due to COVID-19.
- In thromboembolism, a part of the clot (thrombus) breaks off at the site of blood clotting, blocking it with blood flow to a narrower site in the blood vessel.
Dr. Tang et al. Suggest that routine prophylactic, i.e., heparin-induced doses of the disease may reduce mortality in those with the most severe coagulation disorders.6, 7
Dr. Klok et al. Reported an incidence of VTE of 27% and arterial vascular events of 3.7% despite the use of standard weight-based VTE prophylaxis with low molecular weight heparin (LMWH).8 It should be noted that standard VTE prophylaxis in the intensive care unit is 7.7%.9 In addition to the increased incidence of VTE, published pathological reports and industry reports have identified microvascular thrombosis and pulmonary embolism in autopsies in deceased patients.
Pulmonary embolism is most often caused by a blood clot (thrombus) ruptured in another part of the vascular system, which then enters the lung with the bloodstream.
- The incidence of damage to small vessels also increases with aging as plaque buildup in the vessel walls increases so that the vessels become thinner.
- This can be accelerated by high blood pressure, obesity, diabetes, high cholesterol, smoking, sedentary lifestyle, etc., so it can occur with aging regardless of Coronavirus infection.Depending on where it develops or becomes symptomatic, it can cause nervous system problems (imbalance, dizziness, mental decline if it is in the brain), vision problems (if it is in the retina), hearing problems (if it is in the cochlea), and so on.
Microvascular thrombosis is characterized by an environment associated with severe inflammatory lesions, including mononuclear cell infiltrates, virus-infected cells, and diffuse alveolar lesions.10
- Infiltrates show a variation in the tissue stock that results in a denser tissue image, an enlarged pattern.In these areas, the infiltrate may indicate an accumulation of fluid (edema), inflammatory secretions (white blood cells, proteins, foreign substances), or cells (malignant tumors, red blood cells), behind which many diseases may lie.These substances cannot be accurately separated from the lung tissue, which is why the condition is called infiltration.(Home Pharmacy)
Guidance documents from around the world recommend the use of standard coagulation test values to determine the anticoagulant dosage of COVID-19, as recommended by a French guidance document recommending a full therapeutic dose of anticoagulant in patients with fibrinogen levels> 8 g / l or D -dimer level> 3.0 µg / mL.11
While Dr. Klok et al. Reported using 2850 IU nadroparin daily and those over 100 kg received 5700 IU daily, the mean body weight was 87 kg ± 16, and some patients in the study weighed more than 100 kg.
The Dutch centers switched practice after one month of experience in the treatment of COVID-19 patients, in one of the centers the dose of nadroparin was increased to 5700 IU daily, regardless of weight, and in another center it was raised to 5,700 IU twice a day, which is very similar to the increased dose used by Rannuci et al.8, 12 These increased doses are needed to overcome the dramatic increase in procoagulant factors (such as fibrinogen).
- Nadroparin is an anticoagulant that belongs to a class of medicines called low molecular weight heparins.Nadroparin was developed by Sanofi-Synthélabo.Nadroparin is used in general and orthopedic surgery to prevent thromboembolic diseases and to treat deep vein thrombosis.
It can be seen that once the problem persists, it is not easy to remedy, so it is recommended that targeting platelet hyperactivity in the early stages of COVID-19 infection may reduce the thrombotic complications of COVID-19 and reduce the systemic inflammatory response. Reduction of initial platelet activity may be particularly important in higher risk groups.
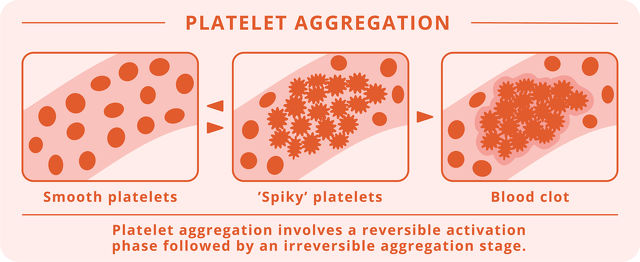
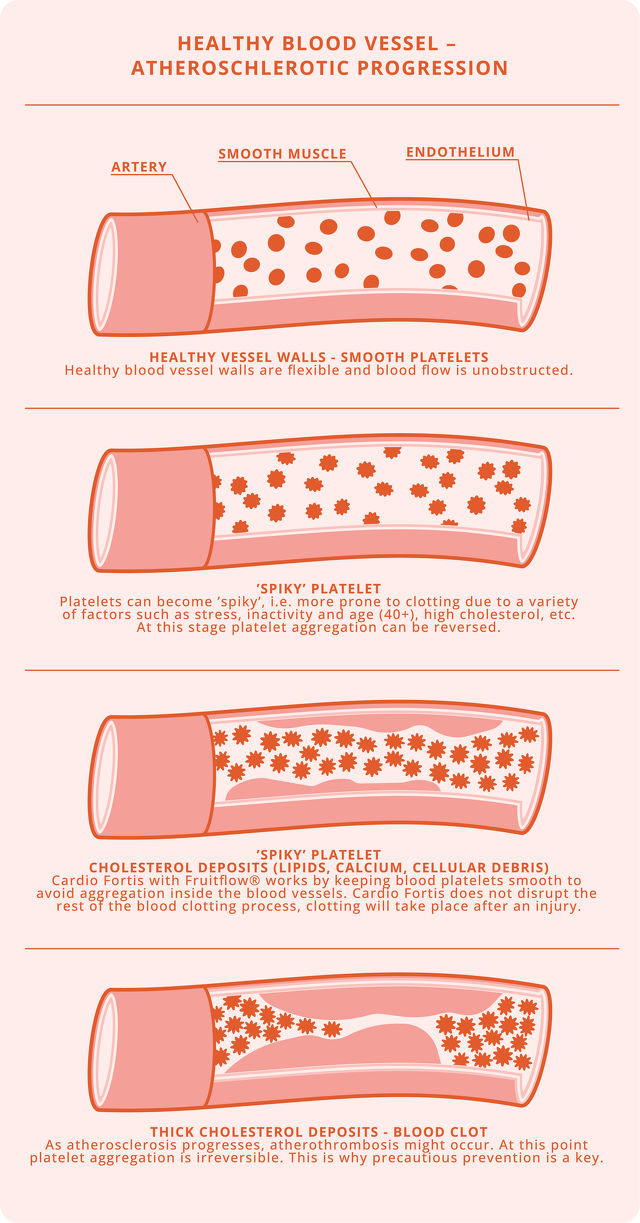
As an alternative to antiplatelet agents, Fruitflow®, a tomato-derived natural dietary supplement to prevent platelet aggregation is considered an appropriate therapy. Fruitflow® contains antiplatelet and anti-inflammatory compounds that target the platelet activation mechanisms characteristic of COVID-19.1
Containing Fruitflow®, rutin and resveratrol, Cardio Fortis is a safe and natural antithrombotic supplement.
Sources
- Platelet hyperactivity in COVID-19: Can the tomato extract Fruitflow® be used as an antiplatelet regime? https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7781513/
- Lillicrap D. Disseminated intravascular coagulation in patients with 2019-nCoV pneumonia. J Thromb Haemost. 2020; 18(4): 786- 787. https://pubmed.ncbi.nlm.nih.gov/32212240/
- Jackson SP, Darbousset R, Schoenwaelder SM. Thromboinflammation: challenges of therapeutically targeting coagulation and other host defense mechanisms. Blood. 2019; 133: 906- 918. https://pubmed.ncbi.nlm.nih.gov/30642917/
- Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020; 395: 1054- 1062. https://pubmed.ncbi.nlm.nih.gov/32171076/
- Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020; 323(11): 1061- 1069. https://pubmed.ncbi.nlm.nih.gov/32031570/
- Tang N, Bai H, Chen X, Gong J, Li D, Sun Z. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost. 2020; 18: 1094- 1099. https://pubmed.ncbi.nlm.nih.gov/32220112/
- Iba T, Levy JH, Warkentin TE, et al. Diagnosis and management of sepsis-induced coagulopathy and disseminated intravascular coagulation. J Thromb Haemost. 2019; 17: 1989- 1994. https://pubmed.ncbi.nlm.nih.gov/31410983/
- Klok FA, Kruip MJHA, van der Meer NJM, et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020; 191: 145- 147. https://pubmed.ncbi.nlm.nih.gov/32291094/
- Lim W, Meade M, Lauzier F, et al. Failure of anticoagulant thromboprophylaxis: risk factors in medical-surgical critically ill patients* Multicenter Study; Randomized Controlled Trial. Crit Care Med. 2015; 43(2): 401- 410.
- Pulmonary and Cardiac Pathology in Covid-19: The First Autopsy Series from New Orleans https://www.medrxiv.org/content/10.1101/2020.04.06.20050575v1
- Traitement anticoagulant pour la prevention du risque thrombotiqe chez un patient hospitalise avec COVID-19 et surveillance de l’hemostase propisitions du ghip et du gfht https://www.fichier-pdf.fr/2020/04/03/covid-19-gihp-gfht-3-avril-final-3/
- Ranucci M, Ballotta A, Di Dedda U, et al. The procoagulant pattern of patients with COVID-19 acute respiratory distress syndrome. J Thromb Haemost. 2020; 18: 1747- 1751. https://pubmed.ncbi.nlm.nih.gov/32302448/